Phases of Pharmaceutical Development Demystified

- 1.
What exactly are the phases of pharmaceutical development?
- 2.
Preclinical research: where the magic (and mice) begin
- 3.
Phase 1 trials: first taste in humans
- 4.
Phase 2 trials: does it actually work?
- 5.
Phase 3 trials: the big leagues
- 6.
Regulatory review: FDA’s final say
- 7.
Phase 4: life after approval
- 8.
How the FDA defines the 4 phases of clinical trials
- 9.
Timeline and cost: why your meds cost a fortune
- 10.
Common myths vs. reality in the phases of pharmaceutical development
Table of Contents
phases of pharmaceutical development
What exactly are the phases of pharmaceutical development?
Ever wondered why it takes like… forever to get a new pill on the shelf? Well, it’s not just bureaucracy gone wild—it’s the phases of pharmaceutical development doing their meticulous dance. From the moment a molecule winks at a scientist in a lab coat to the day your local pharmacy stocks it, the phases of pharmaceutical development are a tightly choreographed marathon, not a sprint. These phases ensure that what ends up in your medicine cabinet is safe, effective, and worth every penny. And trust us, skipping even one step? Big no-no. The whole system’s built on layers—like an onion, or maybe a really complicated lasagna.
Preclinical research: where the magic (and mice) begin
Before any human even sniffs the compound, the phases of pharmaceutical development kick off in petri dishes and test tubes—yep, we’re talking preclinical research. Scientists screen thousands (sometimes millions!) of compounds to find the one that might actually do something useful. Then come the animal studies. No, not your pet hamster—usually rodents or primates. This stage is all about figuring out: does it work? Is it toxic? What’s the right dose? All this data gets bundled up and sent to regulators like the FDA to beg for permission to move to… drumroll… human trials. Without solid preclinical groundwork, the rest of the phases of pharmaceutical development would be like building a skyscraper on quicksand.
Phase 1 trials: first taste in humans
Alright, so now we’re in Phase 1—the first time the drug meets actual humans. Typically, this involves 20 to 100 healthy volunteers (or sometimes patients, if it’s for something like cancer). The main goal? Safety. Researchers are watching like hawks: “Does it cause rashes? Nausea? Spontaneous breakdancing?” Okay, maybe not the last one—but you get the gist. They also test different doses to find the sweet spot. It’s like Goldilocks trying out porridge, except the porridge might give you liver toxicity. Only about 70% of drugs make it past this checkpoint in the phases of pharmaceutical development. So yeah, it’s rough out there.
Phase 2 trials: does it actually work?
Now we’re getting serious. In Phase 2, the drug is tested on a few dozen to a few hundred patients who actually have the condition it’s meant to treat. This is where the phases of pharmaceutical development shift from “Is it safe?” to “Does it work?” Researchers measure efficacy—like, does it lower blood pressure? Shrink tumors? Make your migraines vanish?—while still keeping a close eye on side effects. This phase can last months or even years, and only about 33% of drugs survive it. Fun fact: many promising candidates bite the dust here because they’re just… meh. Not bad, not great—just not worth the risk. And in the high-stakes world of pharma, “meh” doesn’t cut it.
Phase 3 trials: the big leagues
Welcome to Phase 3—the heavyweight division of the phases of pharmaceutical development. We’re talking hundreds, sometimes thousands of patients across multiple countries. This is the final exam before graduation. Regulators like the FDA demand rock-solid proof that the drug works better than existing treatments (or placebo) and that its benefits outweigh the risks. Side effects that were rare in smaller groups now pop up like uninvited guests at a BBQ. If the data looks good? Boom—New Drug Application (NDA) time. But if not? Back to the drawing board, or worse—straight to the trash bin. Only about 25–30% of drugs make it through Phase 3. Ouch.
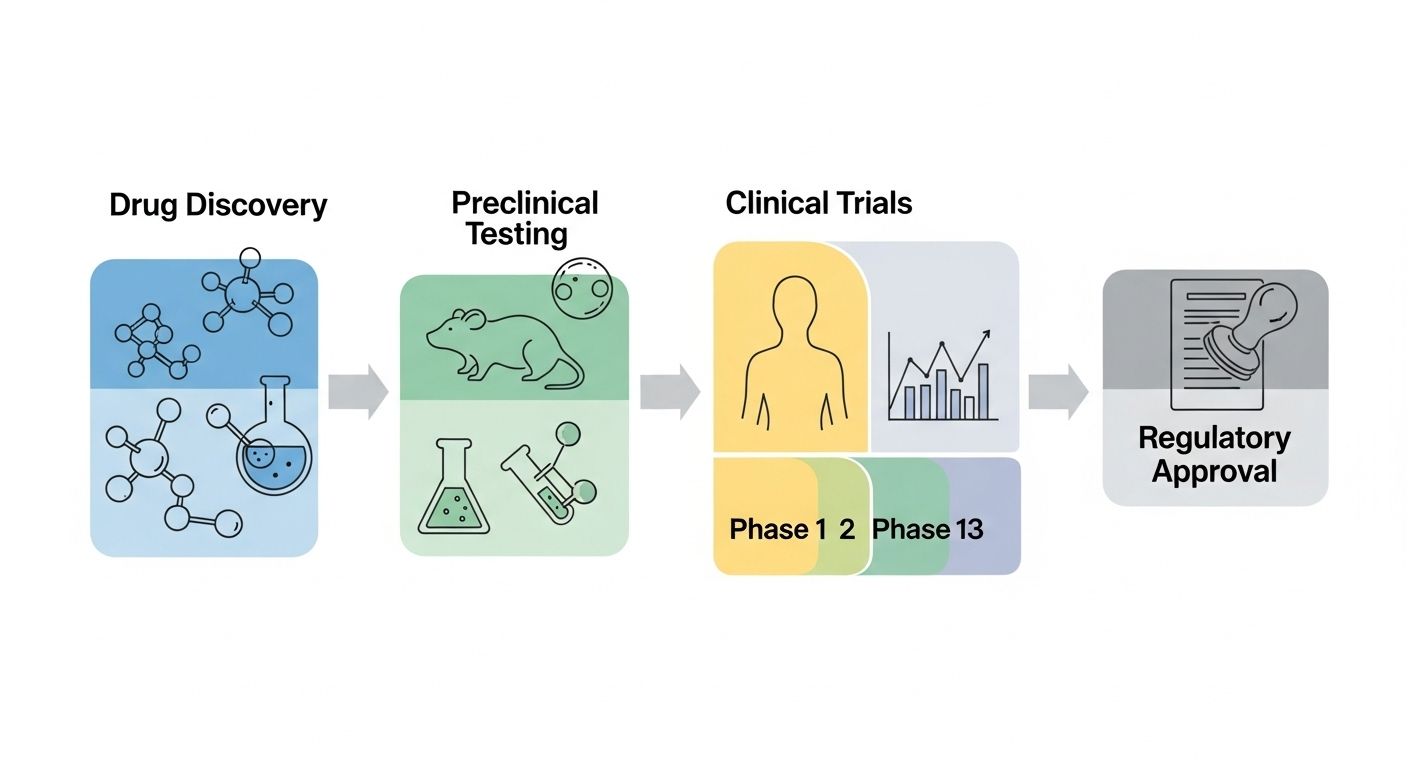
Regulatory review: FDA’s final say
Once Phase 3 wraps up, it’s showtime for the FDA (or EMA, PMDA, etc.). The drug sponsor submits a mountain of data—like, literal truckloads. The FDA then reviews everything: chemistry, manufacturing, clinical results, labeling. This phase can take 6–10 months, or longer if they ask for more info (which they often do). Sometimes they convene an advisory committee—real humans debating real science in real time. If approved, the drug gets a green light. If not? Rejection letter, tears, and maybe a pivot to another indication. This regulatory gate is the climax of the phases of pharmaceutical development, and crossing it means the world to patients waiting for hope.
Phase 4: life after approval
Wait—there’s a Phase 4? Yup! Also called post-marketing surveillance, this is where the phases of pharmaceutical development don’t really end; they evolve. Once the drug’s out in the wild, used by millions, rare side effects might surface. The FDA might require Risk Evaluation and Mitigation Strategies (REMS), or mandate long-term studies. Companies also explore new uses—maybe that arthritis drug also helps with psoriasis? Phase 4 keeps the learning loop alive, ensuring that even after approval, safety and efficacy remain top of mind. Think of it as the “adult supervision” phase of the drug’s life.
How the FDA defines the 4 phases of clinical trials
Let’s clear the air: when folks ask, “What are the 4 phases of clinical trials FDA?”—they’re usually mixing up the full phases of pharmaceutical development with just the clinical part. The FDA recognizes four clinical phases: Phase 1 (safety in small groups), Phase 2 (efficacy + side effects in target patients), Phase 3 (large-scale confirmation), and Phase 4 (post-approval monitoring). But remember—these sit within a broader pipeline that includes discovery, preclinical, and regulatory steps. So while the “4 phases” label is handy, it’s only part of the story. Like quoting one verse and calling it the whole song.
Timeline and cost: why your meds cost a fortune
Here’s the tea: the average drug takes 10–15 years and over $2.6 billion USD to go from lab to pharmacy shelf. Yep, billion. And that’s not just R&D—manufacturing, compliance, failed candidates, and legal fees all add up. Most of that burn happens during the phases of pharmaceutical development, especially Phases 2 and 3. Companies price drugs high not just to profit (though, let’s be real, they do), but to recoup losses from the 90% of molecules that never make it. So next time you side-eye your prescription bill, remember: you’re paying for the one that worked—and the hundred that didn’t.
| Phase | Participants | Duration | Success Rate |
|---|---|---|---|
| Preclinical | N/A (animals/cells) | 1–3 years | ~10% advance |
| Phase 1 | 20–100 | Several months | ~70% |
| Phase 2 | 100–500 | Months–2 years | ~33% |
| Phase 3 | 1,000–5,000 | 1–4 years | ~25–30% |
| Phase 4 | Unlimited (post-market) | Ongoing | N/A |
Common myths vs. reality in the phases of pharmaceutical development
Myth #1: “Drugs are rushed through trials.” Reality? The phases of pharmaceutical development are among the most regulated processes on Earth. Even during emergencies (like the pandemic), standards didn’t drop—they just got faster through parallel processing and emergency funding. Myth #2: “Big Pharma hides side effects.” Actually, Phase 4 exists precisely to catch what earlier trials missed. And regulators can yank approval if new risks emerge (looking at you, Vioxx). The truth is messier, slower, and far more transparent than conspiracy theories suggest. The phases of pharmaceutical development aren’t perfect—but they’re the best system we’ve got for balancing innovation and safety.
For more insights, swing by our Catabasis Pharma homepage, dive into the Development category, or check out our deep-dive piece on phases in drug development you must master. Trust us, it’s worth the read—even if you’re just here for the typos and slang. 😏
Frequently Asked Questions
What are the 4 phases of drug development?
The 4 phases of drug development refer to the clinical trial stages within the broader phases of pharmaceutical development: Phase 1 (safety in small human groups), Phase 2 (efficacy and side effects in target patients), Phase 3 (large-scale confirmation vs. standard care), and Phase 4 (post-marketing surveillance). These sit after preclinical research and before regulatory approval.
What are phase 1, phase 2, and phase 3 trials?
Phase 1 trials test safety and dosage in 20–100 healthy volunteers or patients. Phase 2 evaluates effectiveness and monitors side effects in 100–500 patients with the target condition. Phase 3 confirms efficacy, monitors adverse reactions, and compares the drug to commonly used treatments in 1,000–5,000 patients. All are critical steps in the phases of pharmaceutical development.
What are the steps in the pharmaceutical development process?
The full phases of pharmaceutical development include: (1) Discovery & target identification, (2) Preclinical research (in vitro & in vivo), (3) Investigational New Drug (IND) application, (4) Phase 1–3 clinical trials, (5) New Drug Application (NDA) submission, (6) Regulatory review (e.g., FDA), and (7) Phase 4 post-marketing surveillance. Each step ensures scientific rigor and patient safety.
What are the 4 phases of clinical trials FDA?
The FDA recognizes four clinical trial phases as part of the phases of pharmaceutical development: Phase 1 (initial safety), Phase 2 (efficacy and dose-ranging), Phase 3 (confirmatory large-scale trials), and Phase 4 (post-approval monitoring). These phases are required before and after a drug receives marketing authorization in the U.S.
References
- https://www.fda.gov/drugs/development-approval-process-drugs
- https://www.ncbi.nlm.nih.gov/books/NBK557534/
- https://www.nature.com/articles/nrd.2016.137
- https://www.phrma.org/en/About-PhRMA/Pharmaceutical-Research-and-Development
- https://www.ema.europa.eu/en/human-regulatory/overview/clinical-trials